WATER SOFTENERS
Turn Hard water into Softened water
What ARE WATER SOFTENERS?
Water softeners use a cationic resin which primarily removes dissolved positive mineral salts from the water. The predominant salts and minerals targeted are calcium and magnesium. They turn ‘Hard’ water into ‘Softened’ water. Hard water is the cause of scale and lime deposits that form in pipes, plumbing fixtures and kitchen appliances. Hard water also reacts with soap/detergents and causes a scum which reduces the cleaning capability of the product.
Hardness is primarily caused by the dissolved chemical compounds of calcium and magnesium. The amount of hardness is expressed in mg/l.
Water softeners operate on the principle of ion exchange. Hard water containing positively charged calcium and magnesium ions enters the softener and flows through the negatively charged cation resin bed. The positive ions attach themselves to the negative resin (ion exchange) resulting in the treated or ‘softened’ water to flow out and be used as required.
The Water softener is pre-programmed to regenerate when the resin is saturated. During a regeneration, a salt and brine solution flows into the resin tank and cleans the cation resin by removing the attached deposited minerals. The regenerated water is then flushed out the backwash line.
Benefits of water softeners
- Removes unwanted tastes from water
- Easier on pipes and appliances
- Reduces scaling and lime deposit build-up
- Healthier and softer skin
- Shiny and stronger hair
- Softer, cleaner clothes
- Saves money
Water softener components
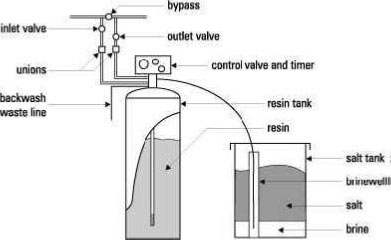
- The Resin Tank: This contains the cation resin
- The Brine or Salt Tank: This contains the salt and saturated brine solution used in regeneration
- The Filter Head: This contains a timer that automatically operates the control valve and is pre-programmed to initiate regeneration
